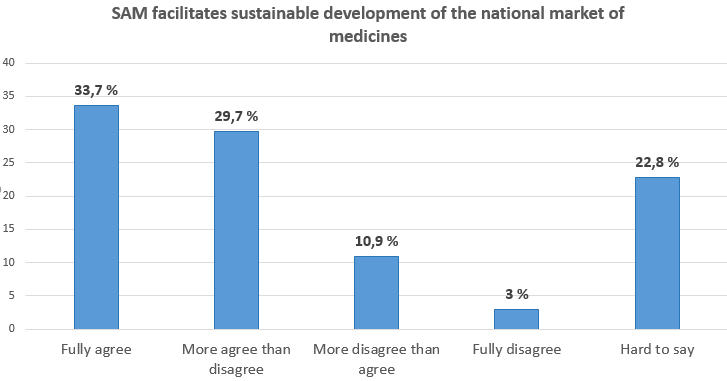
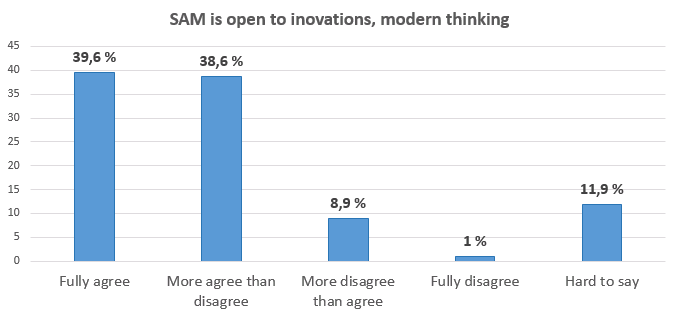
Collaboration partners evaluate the work of the State Agency of Medicines in the annual surveySince 2008 the State Agency of Medicines (SAM) carries out an annual survey of collaboration partners to obtain information regarding the operation of SAM within the year in order to improve the quality of the services provided and make other improvements to the operation of the institution. In October 2015 (13.10.2015. – 30.10.2015.) the survey took place for the 8th time. In this survey participated 101 respondents. The majority of SAM collaboration partners are marketing authorisation holders, medicines manufacturers, medical devices manufacturers, medicines wholesalers, pharmacies, healthcare professionals, medical treatment institutions and research institutions. Basing on the suggestions provided in the previous surveys SAM has introduced new initiatives and has always tried to respect the opinion of clients. For example, the Client Service Centre was established in 2010, electronic circulation of documents is gradually being introduced, informative seminars regarding implementation of normative acts, new European Union requirements and other issues are being organised every year for the collaboration partners. The data from the survey conducted in 2015 indicate that more than half of respondents and in some instances even more than two thirds of respondents completely agree or more agree than disagree that SAM provides qualitative services (93%), SAM e-services facilitate work (89%), SAM helps in understanding requirements of legal acts within the pharmaceutical field (74%), SAM recommendations help in solving complicated issues (78%) and that SAM is open to innovation and a progressively thinking institution (78%). In the survey respondents were asked to evaluate in depth different SAM collaboration directions – particularly the areas in which they have the most intensive everyday collaboration with SAM. Thus, SAM has obtained a detailed evaluation of procedures in all areas of SAM operation: marketing authorisation and variations to medicinal products, pharmacovigilance, clinical trials with medicinal products, import and distribution of medicinal products, registration, vigilance and clinical research of medical devices, licensing and compliance evaluation of pharmaceutical activity companies, compliance evaluation of the procurement of human blood and blood components, tissues, cells and organs.
SAM will use the suggestions and evaluations provided in this survey for further advancement of the institution. This year collaboration partners indicated that it is necessary to "continue the work by maintaining the current professional standards within SAM and in relations with collaboration partners", " more informative seminars are necessary due to the changing environment (processes, systems)", "add the pharmaceutical terms in the database", "broaden the possibilities for electronic communication, for example, receive import and export authorisations", "improve the Medicinal Product Register by expanding the search fields in accordance to the information that is visible upon checking all of the selection criteria", and many more constructive suggestions were made. The State Agency of Medicines expresses its gratitude to the clients for participation in the survey. |
Collaboration partners evaluate the work of the State Agency of Medicines in the annual survey
03.11.2015.